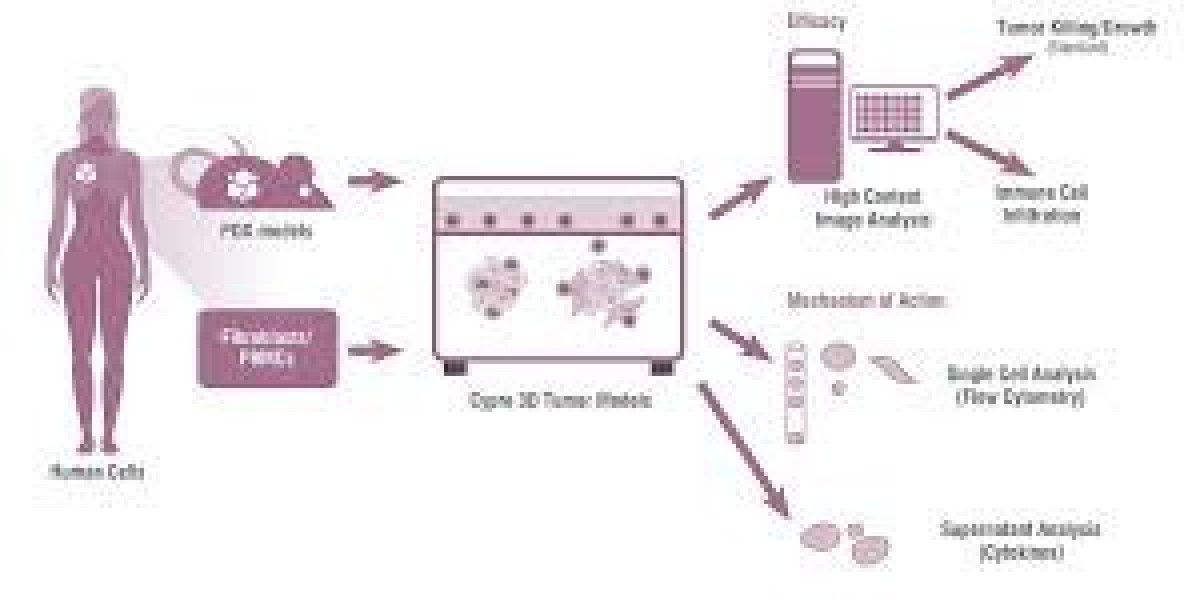
These experimental systems replicate the behavior of tumors, offering a controlled environment to study cancer biology, test therapies, and predict clinical outcomes. Tumor models are pivotal in bridging the gap between laboratory studies and real-world treatment applications.
This article explores the various types of tumor models, their applications, challenges, and the innovations shaping their future.
What Are Tumor Models?
Tumor models are systems designed to mimic the biology of cancer in humans. By replicating aspects of tumor initiation, progression, and response to treatments, these models enable researchers to study cancer's complex behavior and test potential therapies.
They are broadly classified into two categories:
- In Vitro Models – Tumors studied in a controlled, artificial environment, such as cell cultures.
- In Vivo Models – Tumors studied in living organisms, such as animals or humanized systems.
Each type of model serves a unique purpose, and their combined use often provides the most comprehensive understanding of cancer.
Types of Tumor Models
1. In Vitro Tumor Models
In vitro models involve studying cancer cells outside of the body in a laboratory setting. They are essential for early-stage research and high-throughput drug screening.
2D Cell Cultures:
Cancer cells are grown on flat surfaces, such as petri dishes. While widely used for simplicity and scalability, 2D cultures do not capture the three-dimensional complexity of real tumors.3D Cell Cultures:
These models, including spheroids and organoids, grow cancer cells in three dimensions. They better mimic the tumor’s architecture, nutrient gradients, and microenvironment.Organoids:
Miniaturized and simplified versions of organs created from stem cells or tumor cells. Tumor organoids are particularly useful for studying patient-specific cancer behaviors and testing personalized treatments.
2. In Vivo Tumor Models
In vivo models involve studying tumors within living organisms. These models provide critical insights into the interactions between tumors and their microenvironment, immune system, and metastatic processes.
Syngeneic Models:
Tumor cells from the same species as the host are implanted into animals. These models retain a functional immune system, making them ideal for immunotherapy research.Xenograft Models:
Human cancer cells or tissues are implanted into immunodeficient animals (e.g., nude or SCID mice). Xenograft models are invaluable for studying human-like tumor biology and drug responses.Patient-Derived Xenografts (PDX):
Tumors taken directly from patients are implanted into animals. These models maintain the genetic and histological characteristics of the original tumor, making them a key tool in personalized medicine.Genetically Engineered Models (GEMs):
Animals are genetically modified to carry cancer-associated mutations. GEMs allow researchers to study specific genetic drivers of cancer and develop targeted therapies.Humanized Models:
These models involve engrafting human tissues or immune cells into animals, enabling researchers to study human immune-tumor interactions and test immunotherapies.
3. Emerging and Advanced Tumor Models
Advances in technology have led to the development of new models that offer higher precision and relevance.
Tumor-on-a-Chip:
Microfluidic devices that recreate the tumor microenvironment, allowing for precise control of conditions and real-time analysis of tumor behavior.AI-Supported Models:
Artificial intelligence and machine learning are increasingly used to analyze data from tumor models, optimize drug testing, and predict treatment outcomes.
Applications of Tumor Models
Drug Discovery and Development:
Tumor models are essential for identifying new therapeutic targets, testing drug efficacy, and evaluating toxicity. In vitro models allow for high-throughput screening, while in vivo models provide insights into systemic effects and pharmacokinetics.Understanding Cancer Biology:
Tumor models enable researchers to study the mechanisms of tumor initiation, progression, and metastasis. They also provide insights into tumor-immune interactions, angiogenesis, and the role of the tumor microenvironment.Immunotherapy Research:
Models like syngeneic and humanized systems are critical for testing immunotherapies, such as immune checkpoint inhibitors, CAR-T cells, and cancer vaccines.Personalized Medicine:
Patient-derived models, such as PDXs and organoids, are transforming cancer care by allowing researchers to test therapies tailored to an individual’s specific tumor.Studying Metastasis:
Tumor models are invaluable for studying how cancer spreads to other parts of the body and for identifying strategies to prevent or treat metastasis.
Challenges and Limitations
Despite their utility, tumor models face several challenges:
Lack of Full Representation:
No model perfectly replicates the complexity of human tumors. Differences in species (in vivo models) or lack of systemic interactions (in vitro models) can limit their translational relevance.Ethical Concerns:
The use of animals in research raises ethical questions, prompting efforts to develop alternative models and adhere to the 3Rs principle—Replacement, Reduction, and Refinement.Cost and Scalability:
Advanced models like organoids, PDXs, and tumor-on-a-chip systems can be expensive and difficult to scale for widespread use.Time-Consuming Processes:
Developing complex models, such as GEMs or humanized animals, can take significant time and resources.
Future Directions in Tumor Modeling
The field of tumor modeling is evolving rapidly, with several trends shaping its future:
Integration of Technologies:
Combining in vitro, in vivo, and computational models to create hybrid systems that better replicate the complexity of cancer.CRISPR-Based Models:
Using CRISPR-Cas9 technology to create precise genetic modifications in tumor models, improving their accuracy and relevance.AI and Big Data:
Leveraging artificial intelligence to analyze data from tumor models and improve predictions of drug efficacy and patient outcomes.Advanced 3D Models:
Expanding the use of organoids, spheroids, and tumor-on-a-chip systems for more accurate and scalable cancer research.Ethical Alternatives:
Developing non-animal models and refining existing ones to minimize ethical concerns while maintaining scientific rigor.
Conclusion
Tumor models are the backbone of cancer research, enabling groundbreaking discoveries and driving innovations in treatment development. By replicating the complex nature of cancer, these models provide critical insights into tumor biology, drug responses, and patient-specific therapies.
As technologies like CRISPR, AI, and organoids continue to advance, tumor models will become even more sophisticated, offering hope for better cancer treatments and, ultimately, a cure.