The Basic Structure of a Lithium Battery
A lithium battery consists of several key components: the anode, cathode, electrolyte, separator, and current collectors. The anode is typically made of graphite, while the cathode is composed of a lithium metal oxide. These electrodes are immersed in an electrolyte that facilitates the movement of lithium ions. A separator prevents direct contact between the anode and cathode, ensuring safe operation.
The cathode materials vary, with lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), and lithium nickel manganese cobalt oxide (NMC) being among the most commonly used. Each composition offers different trade-offs in terms of energy density, safety, and lifespan. The electrolyte composition, typically consisting of lithium salts dissolved in organic solvents, plays a crucial role in ensuring stable ion movement.
The Chemical Process of Energy Conversion
The energy conversion in batteries, particularly lithium-ion cells, relies on the controlled movement of lithium ions between electrodes. During discharge, lithium ions migrate from the anode to the cathode through the electrolyte, releasing energy that powers external circuits. This process is known as cathode oxidation and anode reduction. When the battery is recharged, an external power source forces lithium ions back to the anode, restoring its stored energy.
As lithium ions travel from the anode to the cathode, electrons simultaneously flow through an external circuit, generating electrical current. This efficient transfer of charge makes lithium-ion polymer and other rechargeable battery systems superior to traditional alternatives.
Role of Electrolyte in Ion Migration
The electrolyte composition plays a crucial role in facilitating ion migration. It typically consists of lithium salts dissolved in an organic solvent, allowing for efficient ion transfer between electrodes. This movement enables lithium-ion polymer and other rechargeable battery systems to maintain high energy efficiency and long cycle life.
Ion migration is carefully regulated by the separator, which allows only lithium ions to pass while blocking electron movement. Advances in electrolyte chemistry, including solid-state electrolytes, aim to further enhance battery safety and performance by reducing the risk of leaks and flammability.
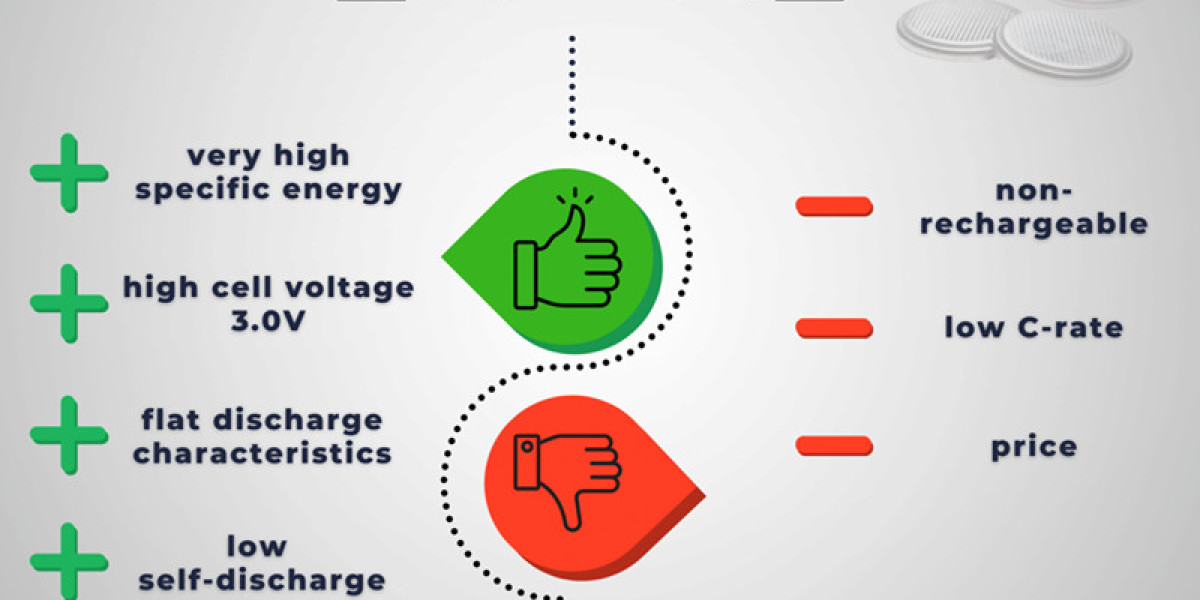
Advantages of Lithium Batteries
Lithium batteries offer several advantages over traditional battery technologies:
High Energy Density: More power is stored in a smaller, lightweight package.
Longer Lifespan: Compared to lead-acid or nickel-based batteries, lithium-ion cells retain their capacity for more charge cycles.
Fast Charging: Lithium batteries can recharge quickly without significant degradation.
Low Self-Discharge Rate: They retain charge longer when not in use.
Eco-Friendly Potential: They eliminate toxic heavy metals found in other battery chemistries, making them a cleaner alternative.
Application Example: LIFMOCER Lithium Battery Technology
LIFMOCER integrates advanced lithium battery technology into energy storage solutions, ensuring optimal performance and reliability. By leveraging superior electrolyte composition and innovative cell designs, LIFMOCER lithium batteries provide enhanced power output and longevity for demanding applications such as forklifts and golf carts.
Future Developments in Lithium Ion Battery Technology
Research into lithium battery advancements continues to push the boundaries of energy storage. Scientists are exploring alternatives like lithium-sulfur and solid-state batteries, which promise higher energy densities and improved safety. Enhanced manufacturing techniques, such as silicon anodes and advanced cathode materials, are also being developed to increase capacity and extend lifespan.
Another innovation is the development of recyclable lithium battery components, reducing environmental impact and supporting sustainability. As demand for electric vehicles, renewable energy storage, and portable electronics grows, lithium battery technology will play a crucial role in the future of energy.
Conclusion
Understanding the lithium battery chemical process is essential for recognizing the benefits of these energy solutions. By efficiently converting chemical energy into electrical power, lithium-ion cells continue to drive advancements in portable electronics, electric vehicles, and renewable energy storage. As technology evolves, innovations in cathode oxidation, anode reduction, and ion migration will further enhance battery efficiency and sustainability.